GRAND RAPIDS, Mich. — Howard Chilson describes himself as a guinea pig, of sorts. He was the first patient to receive a new procedure to treat a specific aortic aneurysm in Michigan.
"I had two choices—that, or being cut open from the back," said Chilson. "And so, we went with this."
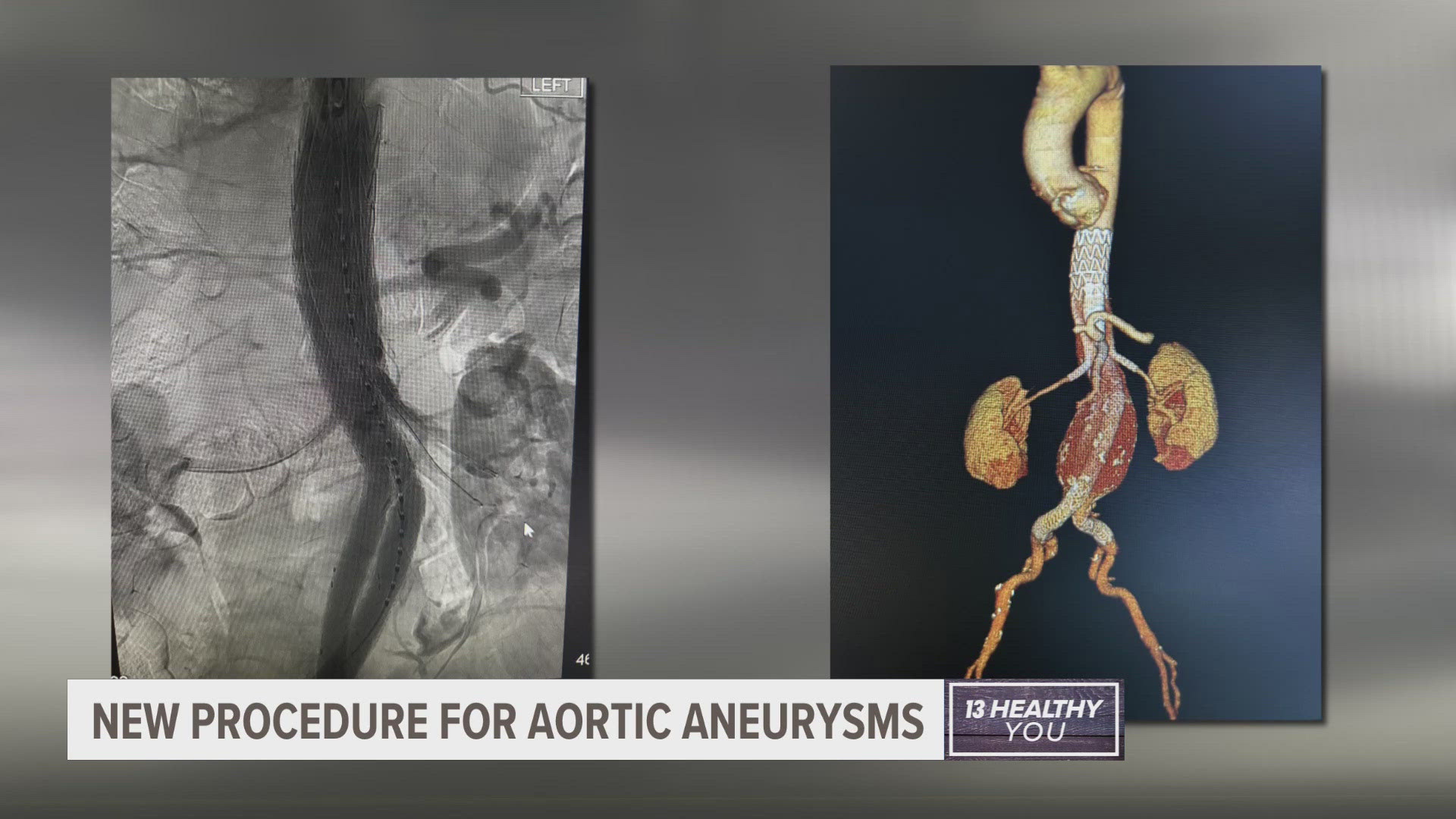
Dr. Eanas Yassa, section chief of vascular surgery at Corewell Health, performed the procedure in September. She used a recently FDA-approved device called GORE® EXCLUDER® Thoracoabdominal Branch Endoprosthesis (TAMBE). The TAMBE device allowed her to treat the aneurysm in a less-invasive way.
"Through only a small incision about the collarbone, and maybe small incisions in the groin, we reconfigure someone's aneurysm," said Yassa. "So that we can take the pressure off it and change their risk of rupture."
The TAMBE is an implantable device the surgeon guides through arteries. It seals off the aneurysm, allowing blood to flow through the device.


Since the procedure, Chilson is doing well, saying with a laugh he's "still kicking."
"I'm not back to 100%, I'm gonna go back in, get a little physical therapy, try to strengthen my legs and stuff," said Chilson. "I think it's a miracle to be able to do that, what they did."
Chilson is also back to his favorite activity: Fishing.
Yassa said she was "so excited" about the new procedure using the TAMBE device. She said traditionally these aneurysms were treated with open surgery, requiring a much longer hospital stay.


"We're hoping that as we get more facile and better at this operation, that we can potentially look at expanding the offerings to more patients," said Yassa.
Corewell Health and Yassa's team currently has more procedures using the TAMBE device planned.